Search Results
Results for: 'Kinetic experiment'
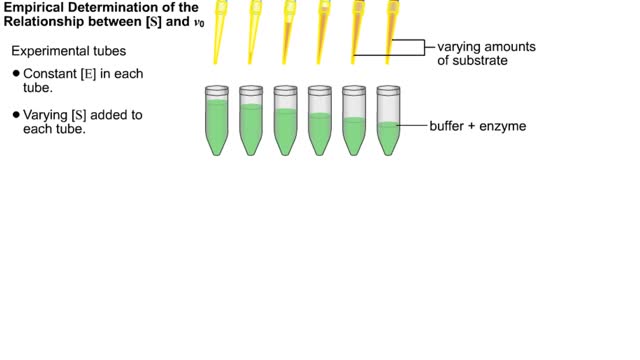
Kinetic parameters & Kinetic experiment
By: HWC, Views: 6498
Kinetics is a measure of the speed or rate of a chemical reaction. A study of kinetics allows us to determine which variables to control (temperature, reactants, catalysts) and how to vary them in order to maximize the amount of products formed and minimize the time involved. Vmax = maximum ve...
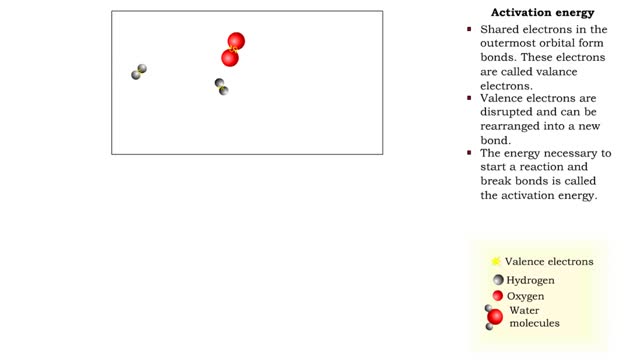
Activation Energy - Valence Electrons
By: HWC, Views: 6103
■ Shared electrons in the outermost orbital form bonds. These electrons are called valence electrons. ■ Valence electrons are disrupted and can be rearranged into a new bond. ■ The energy necessary to start a reaction and break bonds is called the activation energy. ■ Reactants have...
By: HWC, Views: 6017
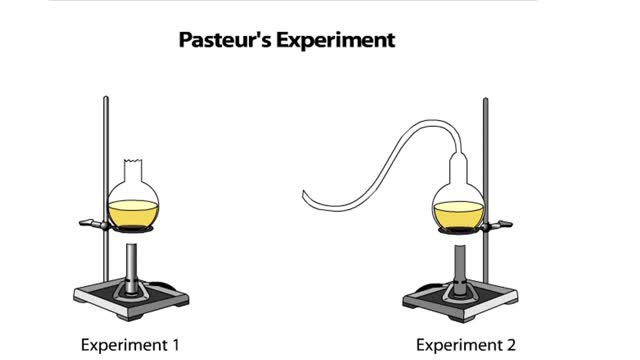
Louis Pasteur designed a procedure to test whether sterile nutrient broth could spontaneously generate microbial life. To do this, he set up two experiments. In both, Pasteur added nutrient broth to flasks, bent the necks of the flasks into S shapes, and then boiled the broth to kill any existing...
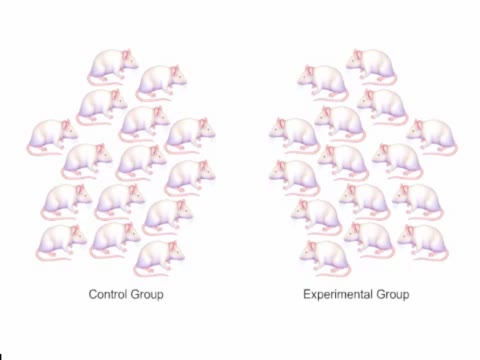
Bacteriophage (Virus) - Mice Experiment
By: HWC, Views: 6389
Also known as phages, these viruses can be found everywhere bacteria exist including, in the soil, deep within the earth's crust, inside plants and animals, and even in the oceans. Bacteriophages (phages) are viruses of bacteria that can kill and lyse the bacteria they infect. ... The lethalit...
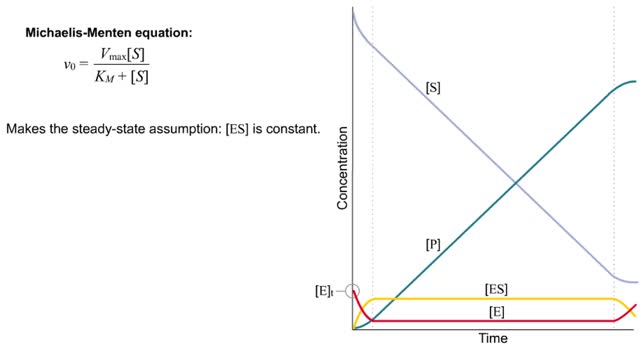
Michaelis–Menten equation & Kinetic parameters
By: HWC, Views: 6391
The Michaelis–Menten equation is the rate equation for a one-substrate enzyme-catalyzed reaction. This equation relates the initial reaction rate (v0), the maximum reaction rate (Vmax), and the initial substrate concentration [S] through the Michaelis constant KM—a measure of the substrat...
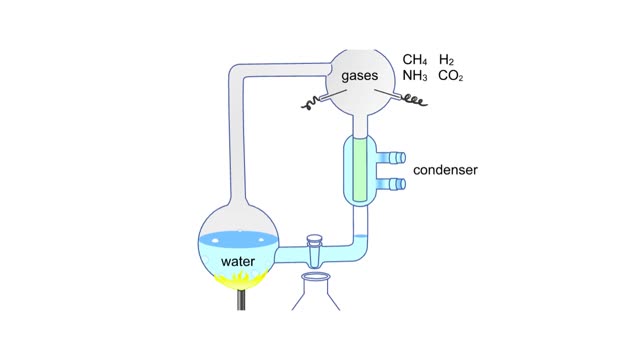
Miller's reaction chamber experiment Animation
By: HWC, Views: 277
A simple diagram of Stanley Miller and Harold Urey's experimental apparatus. The lower portion of the apparatus was filled with water. The upper portion was filled with a mixture of gases that simulated the earth's early atmosphere. Examples are methane, ammonia, hydrogen and carbon dioxide. ...
Photosynthesis and Van Helmont Experiment
By: HWC, Views: 5926
All energy on Earth comes from a star, the Sun. Light must travel 160 million kilometers to reach Earth where plants capture this light energy and convert it to chemical energy in the form of sugars. This biochemical process is called PHOTOSYNTHESIS. The summary equation for photosynthesis is ...
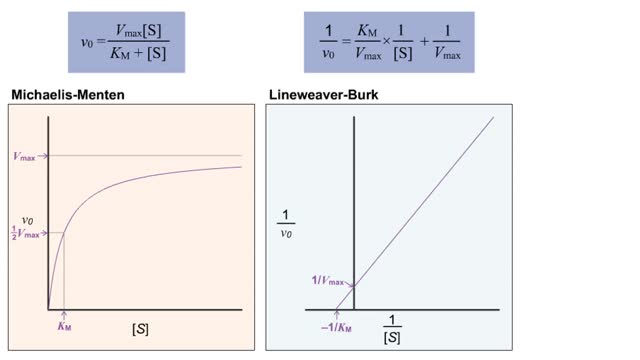
Virtual Enzyme Kinetics & Lineweaver Burk Plot
By: HWC, Views: 6217
• The double-reciprocal (also known as the Lineweaver-Burk) plot is created by plotting the inverse initial velocity (1/V0) as a function of the inverse of the substrate concentration (1/[S]). • This plot is a useful way to determined different inhibitors such as competitive, uncompetitive...
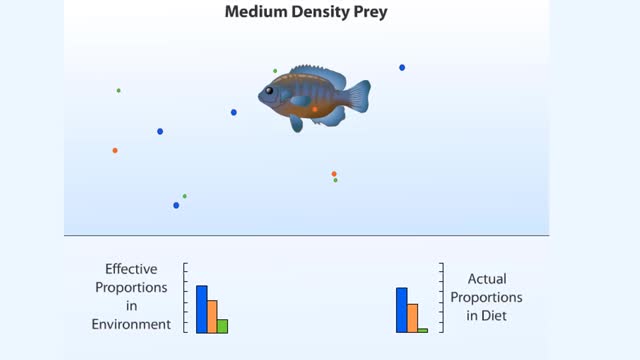
How does an animal choose what food to eat?
By: HWC, Views: 6135
One might assume that natural selection has influenced the foraging behaviors of animals, and that most animals forage efficiently, spending the least energy to gain the most nutrients. This is the underlying assumption of optimality modeling, a scientific approach to studying foraging behavior. ...
Advertisement